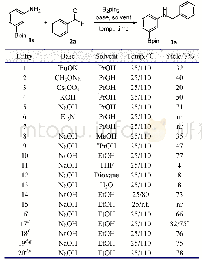
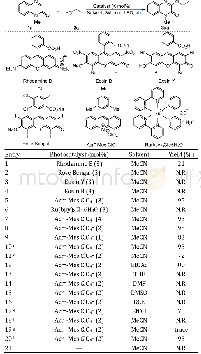
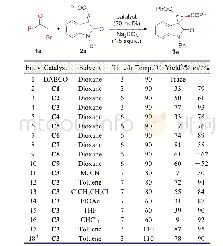
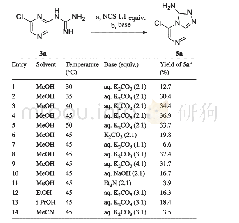
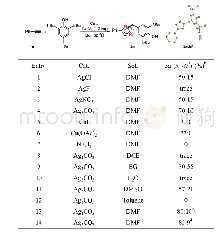
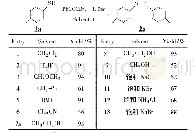
《Table 2 Optimization of the reaction conditions》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《2-吡唑啉-9-芳基邻菲咯啉镍配合物催化芳基卤化物和格氏试剂偶联反应的研究(英文)》
a.Reaction conditions:aryl bromide 1.0 mmol,PhMgBr 1.2 mmol,solvent 5mL;b.GC yield;c.reflux
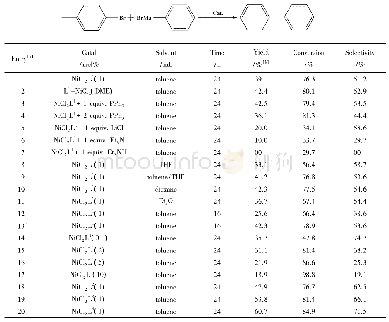
With these considerations in mind,our initial work focused on the model reaction of p-MeC6H4Br with PhMgBr to optimize the reaction conditions.These results are summarized in Table 2.With toluene as the solvent and at room temperature,we first examined the effect ofcatalyst precursors.To our delight,the C—C coupling reaction of p-MeC6H4Br with PhMgBr could proceed smoothly in the presence of Ni Cl2L1complex(Table 2,entry 1).To achieve better results,various commercially available salt additives[33]were introduced to these systems,and the results proved that 1 equiv.PPh3was the best choice(Table 2,entry 3).Meanwhile,we conducted the experiment in situ by using ligand L1and(DME)Ni Cl2,turning out to be the yield of p-MePh-Ph as well as Ni Cl2L1complex(Table 2,entry 2).Subsequently,different solvents(non-polar or polar solvents)were examined.The results showed that toluene and dioxane were proved to be the best for the formation of p-MePh-Ph.After these optimized conditions were developed,the effects of dosage of Ni Cl2L1complex and temperature were studied as well.Investigation of temperature revealed that getting the same yield needed to shorter time.Besides,with either an increase or decrease of the amount of the Ni Cl2L1complex,inferior results were observed.
| 图表编号 | XD0018900100 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.10.01 |
| 作者 | 崔凤娟、王环宇、邓庆芳、江艳、曹艳珍、赵桦萍 |
| 绘制单位 | 齐齐哈尔大学化学与化学工程学院、齐齐哈尔大学化学与化学工程学院、齐齐哈尔大学化学与化学工程学院、齐齐哈尔大学化学与化学工程学院、齐齐哈尔大学化学与化学工程学院、齐齐哈尔大学化学与化学工程学院 |
| 更多格式 | 高清、无水印(增值服务) |