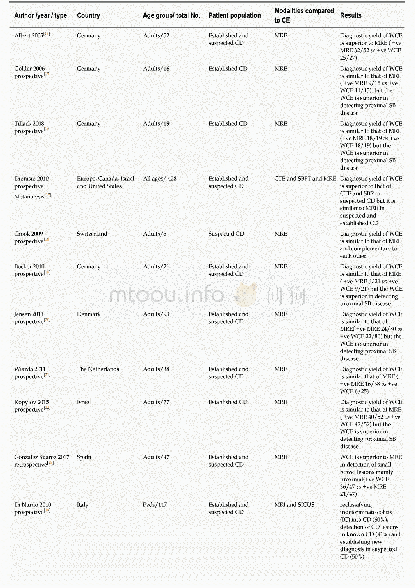
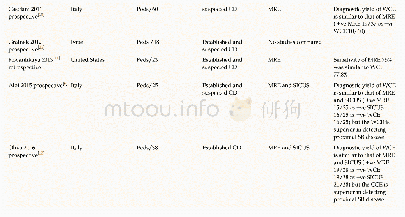
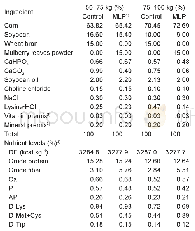
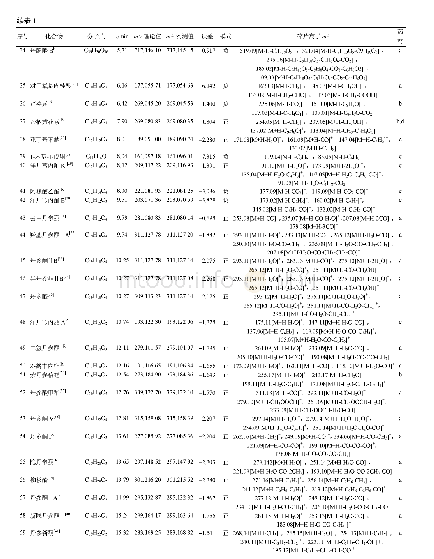
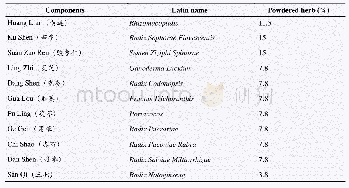
《Table 2 Components of DXR (Capsule ingredients)》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《定心方联合胺碘酮治疗室性早搏:一项随机、双盲、多中心、安慰剂对照试验(英文)》
All herbs for DXR were purchased from the Affiliated Nanfang Hospital of Southern Medical University and were available over-the-counter throughout China.No product used in this trial was an animal product,endangered species or controlled substance.All herbs were administered in the dried powdered form and encapsulated.The dried powdered DXR was prepared by the principal supplier,YiJia Pharmaceutical Company,Guangzhou,China(Table 2).The DXR placebo was indistinguishable from the DXR in form,color,smell,taste,size and packaging after testing on10 independent volunteers.DXR and the placebo formulation were supplied in the same opaque capsules.The principal supplier was also contracted to pack the drug(amiodarone 200 mg tablets)and matching placebo tablets.Patients in the DXR group and the DCA group were required to take 5 capsules twice per day for 8 weeks.The dose of amiodarone or amiodarone placebo was 600 mg/day orally in three divided daily doses for 2 week,then 400 mg/day orally in two divided daily doses for 4 weeks followed by a maintenance oral dose of 200 mg/day for 2 week.Compliance was assessed by pill count.
| 图表编号 | XD0014716300 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.03.01 |
| 作者 | 周凤华、贾钰华、叶遂林、娄林洁、杨平、李杰、张月均、钱荣江 |
| 绘制单位 | 南方医科大学、南方医科大学、广州中医药大学、温州大学第一附属医院、江西中医药大学附属中西医结合医院、山西省中医院、横店市人民医院、宁海县中医院 |
| 更多格式 | 高清、无水印(增值服务) |